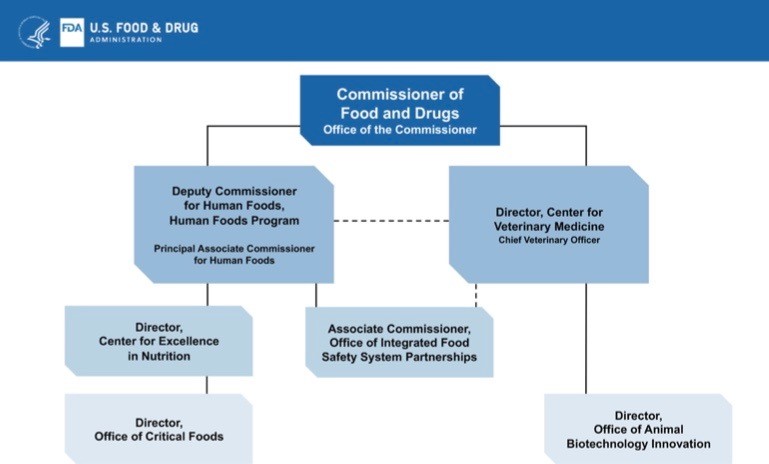
The US Food and Drug Administration has announced a redesign of its Human Foods Program to focus on protecting and promoting a safe, nutritious U.S. food supply that more quickly adapts to an ever-changing and evolving environment, while centralizing authority under a single leader, a deputy commissioner, who reports directly to the FDA commissioner.
CDFA Secretary Karen Ross: “CDFA appreciates its partnership with FDA on a number of issues, especially food safety and the implementation of the Food Safety Modernization Act. I applaud Commissioner Califf’s announcement today creating the FDA Human Foods Program. His vision for a unified Human Foods Program with a single leader reporting directly to the Commissioner unifies the important functions of food safety and nutrition which should result in an agile, effective program. I am excited about the proposal to create a Center for Excellence in Nutrition to help consumers with the information they need to make better food choices for health and quality of life, as well as the establishment of an Office of Integrated Food Safety System Partnerships as an acknowledgement of the state and local regulatory partners who share a passion for protecting food safety and consumer protection.
“Organizational change is challenging and takes time. This is just the first step. FDA has talented, dedicated staff and I am optimistic the formation of an Implementation and Change Management Group will include seasoned professionals as well as the next generation of leaders who can help develop the structure, appropriate lines of authority, adequate resources, and technologies to make FDA an even better consumer protection agency.”